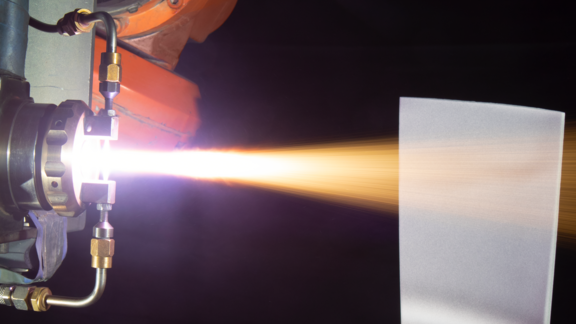
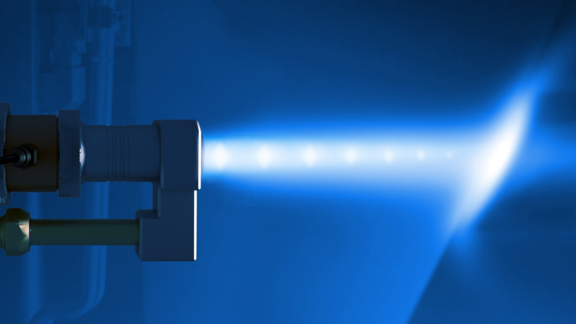
At elevated temperatures, an electrolyte is not required to drive the corrosion reaction. Instead, ions within hot gases attack the substrate directly. In high-temperature oxidation, it is oxygen that causes the attack through the formation of an oxide scale on the surface of the component. Initially, the scale may be relatively stable, but as time goes on, the scale continues to grow. Stresses build within the scale and eventually, the stresses become high enough to cause the scale to crack and spall. The process then repeats to the point of failure.
Sulfidation is a similar mechanism to that of oxidation, but the corrosive culprit comes from the presence of sulfur compounds. Sulfidation can be more aggressive than oxidation because these sulfur based scales are less stable than oxides.